
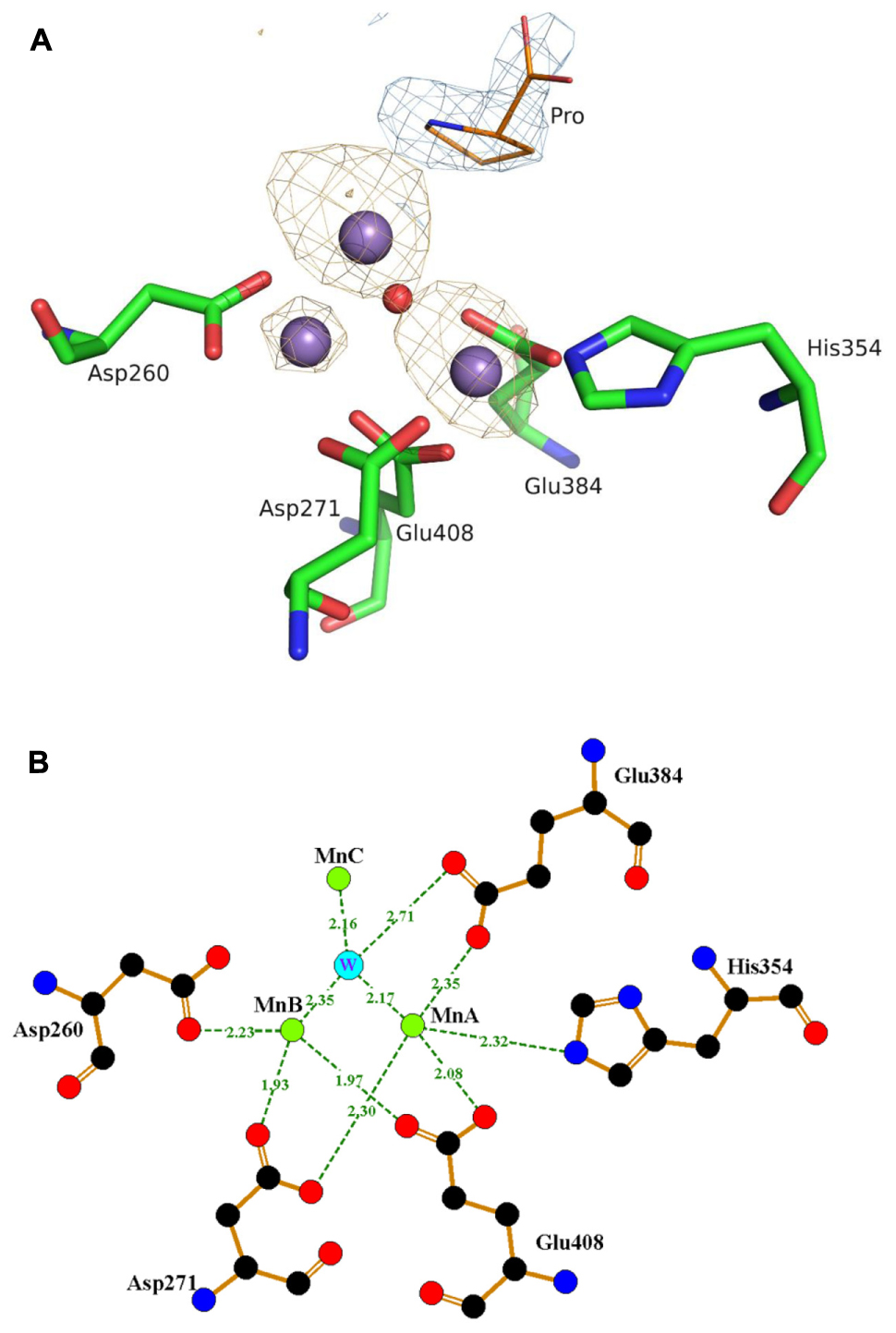
Punitha Vedantham, Mianji Zhang, Parul J.Dicobalt II−II, II−III, and III−III Complexes as Spectroscopic Models for Dicobalt Enzyme Active Sites. Bond, Ulla Gro Nielsen, Boujemaa Moubaraki, Keith S. Discovery of Inhibitors of Escherichia coli Methionine Aminopeptidase with the Fe(II)-Form Selectivity and Antibacterial Activity. Discovery, Identification, and Characterization of Candidate Pharmacodynamic Markers of Methionine Aminopeptidase-2 Inhibition. Kinetic and Spectroscopic Analysis of the Catalytic Role of H79 in the Methionine Aminopeptidase from Escherichia coli. Magnetic Circular Dichroism Study of a Dicobalt(II) Methionine Aminopeptidase/Fumagillin Complex and Dicobalt II−II and II−III Model Complexes. Magnetic Circular Dichroism Study of a Dicobalt(II) Complex with Mixed 5- and 6-Coordination: A Spectroscopic Model for Dicobalt(II) Hydrolases. Catalysis and Inhibition of Mycobacterium tuberculosis Methionine Aminopeptidase. Protein N-Terminal Processing: Substrate Specificity of Escherichia coli and Human Methionine Aminopeptidases. Pyridinylquinazolines Selectively Inhibit Human Methionine Aminopeptidase-1 in Cells. Meyers, Karen Tenney, Joong Sup Shim, Phillip Crews, L. Feiran Zhang, Shridhar Bhat, Sandra B.Journal of the American Chemical Society 2015, 137 Generation, Characterization, and Tunable Reactivity of Organometallic Fragments Bound to a Protein Ligand. Targeting Metalloenzymes for Therapeutic Intervention. Discovery and Structure-Based Optimization of Next-Generation Reversible Methionine Aminopeptidase-2 (MetAP-2) Inhibitors. Timo Heinrich, Jeyaprakashnarayanan Seenisamy, Beatrix Blume, Jörg Bomke, Michel Calderini, Uwe Eckert, Manja Friese-Hamim, Rainer Kohl, Martin Lehmann, Birgitta Leuthner, Djordje Musil, Felix Rohdich, Frank T.Identification of Methionine Aminopeptidase-2 (MetAP-2) Inhibitor M8891: A Clinical Compound for the Treatment of Cancer. Timo Heinrich, Jeyaprakashnarayanan Seenisamy, Frank Becker, Beatrix Blume, Jörg Bomke, Melanie Dietz, Uwe Eckert, Manja Friese-Hamim, Jakub Gunera, Kerrin Hansen, Birgitta Leuthner, Djordje Musil, Jens Pfalzgraf, Felix Rohdich, Christian Siegl, Dieter Spuck, Ansgar Wegener, Frank T.This article is cited by 85 publications. We conclude that MetAP2 is a manganese enzyme and that therapeutic MetAP2 inhibitors should inhibit MetAP2-Mn 2+. In cell culture assays, A-310840 did not inhibit intracellular MetAP2 enzyme activity and did not inhibit cell proliferation despite its ability to permeate and accumulate in cytosol, while A-311263 inhibited both intracellular MetAP2 and proliferation in a similar concentration range, indicating cellular MetAP2 is functioning as a manganese enzyme but not as a cobalt, zinc, iron, or nickel enzyme. In contrast, A-311263 inhibited MetAP2 with Mn 2+, as well as Co 2+, Fe 2+, Ni 2+, and Zn 2+. A-310840 below 10 μM did not inhibit the activity of MetAP2−Mn 2+ but was very potent against MetAP2 with other metal ions including Co 2+, Fe 2+, Ni 2+, and Zn 2+ in the in vitro enzyme assays. To determine which metal ion is physiologically relevant, we then tested inhibition of intracellular MetAP2 with synthetic inhibitors selective for MetAP2 with different metal cofactors. In the presence of reduced glutathione to mimic the cellular environment, Co 2+ and Mn 2+ were also the best stimulators (∼30-fold) for MetAP2 enzyme activity. The activity of MetAP2 on either MAS or MGAQFSKT was enhanced 15−25-fold by Co 2+ or Mn 2+ metal ions in a broad concentration range (1−1000 μM). To examine this question, we first investigated the effect of eight divalent metal ions, including Ca 2+, Co 2+, Cu 2+, Fe 2+, Mg 2+, Mn 2+, Ni 2+, and Zn 2+, on recombinant human methionine aminopeptidase apoenzymes in releasing N-terminal methionine from three peptide substrates: MAS, MGAQFSKT, and 3H-MASK(biotin)G. The identity of the physiological metal cofactor for human methionine aminopeptidase-2 (MetAP2) has not been established.


 0 kommentar(er)
0 kommentar(er)
